From Terminator to Exterminator
Biotechnologists have a child-of-terminator technology, that could have even more far-reaching consequences, called ‘gene drives’,
- Análisis
| Article published in ALAI’s magazine No. 542: Social justice in a digitalized world 24/06/2019 |
It has been twenty years since ETC Group uncovered a US patent on what became known as ‘terminator technology’ – seeds genetically engineered to stop farmers breeding from them[1]. Civil society and farmer movements protested that such ‘suicide seeds’ would threaten seed-saving practices that are as old as agriculture[2].
The story of Terminator became iconic in the global battle over genetically modified organisms (GMOs). Only interested in protecting their profits, its developers failed to assess the potential social, economic and environmental impact of engineering sterility. Following an uproar from across society, including United Nations bodies, Terminator was placed under a global moratorium under the UN’s Convention on Biological Diversity (CBD) in 2000.
Terminator was part of ‘first-generation’ GM crops, which involved altering common crops to be resistant to pests (such as cotton bollworm) or weed-killers (such as Bayer-Monsanto’s Roundup). GM crops ran into problems when many consumers didn’t buy foods grown using GM and farmers found the promised benefits only materialised, if at all, in the short-term.
Realising that their attempts at achieving public acceptance had got off to a bad start, biotech firms such as Syngenta (now part of ChemChina), proposed a second generation of GM crops that would have clearly defined benefits. The public relations coup was meant to be a variety of rice produced through genetic engineering to biosynthesize a precursor of vitamin A. However, this ‘golden rice’ was not the ‘magic bullet’ solution that its promoters claimed. In 2008, WHO (World Health Organization) malnutrition expert Francesco Branca cited the lack of real-world studies and uncertainty about how many people will use golden rice, concluding "giving out supplements, fortifying existing foods with vitamin A, and teaching people to grow carrots or certain leafy vegetables are, for now, more promising ways to fight the problem"[3].
Now biotechnologists have a child-of-terminator technology, that could have even more far-reaching consequences called ‘gene drives’. Where Terminator allowed companies to render their own proprietary seeds sterile, gene drive organisms (GDOs) go further – actively and invasively spreading engineered genes into the wild. GDOs potentially pose a far more dangerous threat to people’s rights, food security and the environment than Terminator ever did.
Exterminator drives
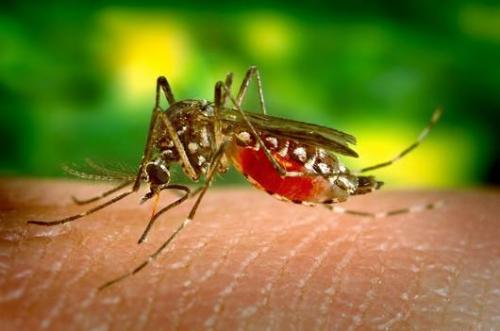
Gene drives appear to only be operational in laboratory settings, so far, and are designed to be invasive and persistent in natural ecosystems. GDOs are genetically engineered to make them take over and then potentially eliminate entire populations. We should call them what they are: ‘exterminator drives’. Since their first emergence in 2014, GDOs have become a public relations poster-child for the biotech industry to re-launch itself as socially useful. It has become an increasingly important investment vehicle, as GM-free markets boom and consumer lawsuits against the industry’s previous innovations proliferate[4]. While scientists promoting GM crops used golden rice to claim the moral high ground, those promoting gene drives claim they could help end an even bigger global killer – malaria. Through a project called Target Malaria, led by Imperial College in London in the UK, US$100 million is being directed to gene drive research, with the release of the first GMO mosquitos in 2018/19 being followed by GDO mosquitos in West African villages; the promise is that the technology will soon eliminate one the world’s most deadly infectious diseases.
Calls for the use of GDOs to tackle malaria often ignore the kind of well-proven techniques that have eradicated the disease in scores of countries, most recently in Paraguay and Sri Lanka. Target Malaria’s GDOs are being promoted as a vital ‘tool in the toolbox’ against the disease, whereas in fact they would be a high-stakes gamble with the ecology of food systems and biodiversity across the planet.
While first-generation GMOs mostly spread engineered genes by accident, GDOs will be designed to do their own engineering among wild populations out in the real world. Their spread to those populations would be deliberate. Scientists behind GDOs have only just begun to ask what would happen if the genes aren’t quite as well behaved as their Mendelian models intended. What if genes for female sterility, for instance, which have been shown to eliminate mosquito populations in the lab, transferred to species that pollinate our crops or are a food source for birds, reptiles, even humans? What if genes that were beneficial became disabled, or if genetic disruption increased the prevalence or altered patterns of diseases?
Farm Gates
Multi-million-dollar grants for gene drive development from the Bill and Melinda Gates Foundation, the Foundation of National Institute of Health, the Open Philanthropy Institute, The Wellcome Trust and the US Defense Advanced Research Projects Agency have included generous allowances for public message testing, public engagement exercises, lobbying and communications activities. One initiative, the ‘Gene Drive Outreach Network’, curiously fails to mention any proposed agricultural uses of gene drives in its factsheets, focusing only on ‘global health’ and ‘conservation’ uses[5].
This omission of agricultural uses in the promotion of GDOs is not accidental. It fits exactly with the priorities expressed by gene drive pioneers such as Kevin Esvelt of the Massachusetts Institute of Technology (MIT), who holds one of two key foundational patents on gene drives. More than a quarter of his 38-page patent is taken up describing agricultural applications for the technology. Yet, in conversation with one of the authors (Thomas) in 2016, Esvelt commented that agricultural applications should wait on public health and conservation applications simply because the benefits are not as clear to ordinary citizens. He also commented that it would be a bad idea to talk publicly about the agricultural uses listed in his patent, such as reversing herbicide resistance in weeds, because it would only benefit Monsanto (now Bayer).
GDO developers may be warning agribusiness and each other to keep a low profile on gene drives but that is not to say agribusiness is not actively engaging on the topic. From internal communications obtained by civil society organizations through access to information laws in the US, officials of the former Monsanto were closely in touch with military scientists in 2017 on classified study on gene drives[6]. Agribusiness majors, including Syngenta (now owned by ChemChina) and Dow Agroscience (now Corteva), have also been closely involved in US Gene Drive policy discussions[7].
Global genetic force-feeding
Releasing limited local or targeted gene drive organisms as a service may be the most obvious business model for agricultural use, but making money from ‘global drives’ may also be possible for gene drive companies. Esvelt and others have proposed that so called ‘sensitizing’ gene drives could be released into weed or pest species that make those species susceptible to a particular chemical compound, such as an herbicide or pesticide – for example re-sensitizing pigweed (Amaranthus palmeri) to Monsanto’s Roundup (glyphosate) or to a new proprietary chemical. This approach would enable the manufacturer of the compound (in this case Bayer) to sell their proprietary chemical as perfectly matched to the wild weed species. Whereas the former Monsanto previously made its crop seeds ‘Roundup-ready’ (that is, resistant to glyphosate) to boost glyphosate sales, now it is the weed itself that becomes ‘ready’ to wilt in response to Roundup. When weeds are not totally eradicated, they may evolve to become resistant once again to the herbicide of interest. In such a situation, the gene drive is only a temporary solution and would have to be applied repeatedly.
Challenges for policy-makers
While gene drive developers claim that there may be ways to effectively contain GDOs in the future, these hypothetical claims and assumptions need to be rigorously examined and tested. Strict laboratory handling and containment rules for all gene drive research must be internationally agreed and put into practice before further research can proceed, even in the lab. At present, it appears possible for scientists to develop new GDOs without them being subject to any specific biosafety regulations. In some jurisdictions, such as Brazil, it is not even clear whether they will be subject to the weak biosafety rules that controlled the development and use of GMOs.
Technologies that originate in the laboratory, such as GMOs and now GDOs, ignore deep-seated injustices and power imbalances which require political answers and democratic scrutiny, rather than technical quick-fixes. At both national and international levels, questions of technology assessment and societal consent have only begun to be formally addressed since pressure was put on by grassroots-based and other civil society organisations.
The recent meeting of parties to the CBD in Egypt recognises the serious risks and ‘uncertainties’ around the gene drive technology[8]. It calls upon governments only to consider introducing gene drive organisms into the environment for experimental research, when “scientifically sound case-by-case risk assessments have been carried out,” when “risk management measures are in place to avoid or minimize potential adverse effects” and when the “free, prior and informed consent” of “potentially affected indigenous peoples and local communities is sought or obtained”.
The outcome of these negotiations places consent at the heart of any path toward the potential release of gene drive organisms, which has put the spotlight back on the adequacy of Target Malaria processes for gaining consent in villages in Burkina Faso where they are scheduled to soon release ‘male sterile’ genetically modified mosquitoes as a preliminary step towards releasing others with gene drives. Target Malaria argue that “it’s not logistically possible to obtain consent from each and every person affected” when it comes to GM mosquitoes. However, when it comes to such a controversial technology, with potentially serious ecological effects and as yet unknown consequences for health, giving consent cannot be limited to a handful of residents.
Today it is Burkina Faso that is being force-fed gene drives. Yet, decisions taken in this African state in relation to this exterminator technology could set an international precedent. Proposals to release gene drive organisms on indigenous territories in New Zealand[9], Australia[10] and Hawaii[11] are on the agenda for the coming months. The world needs to ask how genuinely release proponents are seeking to obtain “free, prior and informed” consent and what rights people will have to say yes or no.
Neth Daño, Jim Thomas and Tom Wakeford, Action Group on Erosion, Technology and Concentration (ETC Group).
[2] See resources at: http://www.etcgroup.org/issues/terminator-new-enclosures (in English) and http://redtecla.org/ (in Spanish) and http://www.etcgroup.org/fr/content/des-technologies-terminator-aux-technologies-exterminator (in French).
[3] Enserink, Martin. “Tough Lessons From Golden Rice.” Science 320, no. 5875 (April 25, 2008): 468–71.
[4] See: https://uk.reuters.com/article/us-bayer-glyphosate-lawsuits/bayers-monsanto-faces-8000-lawsuits-on-glyphosate-idUKKCN1L81J0
[7] A Feb 2016 workshop to develop a roadmap on gene drive research included the international policy lead for Syngenta, Tichafa Munyikwa. On another occasion, discussions included Steven Evans of Dow Agrosciences.
Del mismo autor
- From Terminator to Exterminator 17/07/2019
- De Terminator a "Exterminator" 12/07/2019
Clasificado en
Clasificado en:
Ciencia y tecnología, Internet ciudadana
- Nick Bernards 31/03/2022
- Paola Ricaurte 10/03/2022
- Burcu Kilic 03/03/2022
- Shreeja Sen 25/02/2022
- Internet Ciudadana 16/02/2022

