Abdala and Soberana 02 vaccines near the finish line
The Abdala vaccine showed 92.28% efficacy with three doses during the third phase of clinical trials and the Soberana 02 vaccine showed 62% efficacy with just two of its three doses
- Información
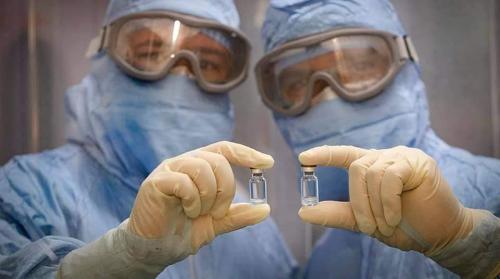
Cuban state-owned pharmaceutical company BioCubaFarma, on June 21, reported that the country’s locally developed anti-COVID-19 vaccine candidate, Abdala of the Center for Genetic Engineering and Biotechnology (CIGB), showed 92.28% efficacy with three doses during the third phase of clinical trials. The significant news came two days after the news that another vaccine candidate, Soberana 02 of the Finlay Vaccine Institute (IFV), showed 62% efficacy with just two of its three doses during the phase 3 clinical trial.
BioCubaFarma highlighted that Cuba now has “two vaccines that meet the minimum requirements for clinical efficacy declared by the World Health Organization (WHO).” The historic achievements were hailed by scientists, political leaders, organizations and people across Cuba.
Cuban President Miguel Díaz-Canel personally visited the CIGB’s headquarters and attended the event where the results were announced and the celebration began. In a series of tweets, the head of the state expressed the sense of heightened joy and pride that the whole country has been feeling since yesterday.
“In less than 48 hours, Cuban scientists have given our heroic people, who are resisting the criminal blockade that is intensified in a pandemic, two overwhelming results. In two days, we denounced the blockade to the world by having our own two vaccines,” tweeted Díaz-Canel.
“Struck by two pandemics (COVID-19 and blockade), our scientists from Finlay and CIGB have jumped over all obstacles and have given us two very effective vaccines: Soberana 02 and Abdala,” he wrote in another tweet.
The president also recalled the revolutionary words of national hero José Martí and the revolutionary ideology of Commander Fidel Castro.
“I think of Martí, who wrote that ‘it is not the received and casual intelligence that gives the man honor; but the way he uses it and saves it. There is only one way to endure and that is to serve’. And above all, I think of Fidel, who did so much for Science and Medicine at the service of Humanity. He said: “’veryone who has a vocation for health, I invite you to study’…There is a huge world that is in need,” tweeted Díaz-Canel.
The phase III clinical research for Abdala began on March 22 with the participation of 48,290 volunteers. They were administered three doses at an interval of three weeks. The process to evaluate the efficacy began on May 3.
The third phase of clinical trials for Soberana 02 began on March 4 with 44,010 volunteers between the ages of 19 and 80. They were administered two doses of Soberana 02 and a third booster shot of Soberana Plus over 56 days.
On June 19, the director of the IFV, Vicente Vérez Bencomo, announced that the results of the efficacy study of Soberana 02 with a third booster dose would be available in a couple of weeks. The data on the quality and quantity of antibodies generated with a third dose constitutes the final step to present the research to the regulatory authority and validate it as a vaccine.
The IFV also announced that it would soon request the country’s Center for State Control of Medications, Medical Equipment and Devices (CECMED) to authorize the emergency use of the vaccine as it already exceeds the 50% efficacy threshold required for approval by the WHO.
The results obtained after the administration of two doses of Soberana 02 are fairly positive. The vaccine has also shown efficacy against the original strain, as well as against the combination of mutant strains of the virus that are circulating in the country.
President Díaz-Canel, while celebrating the news during the weekend, stressed that “the wait will not be long. Nor can it deprive us of celebrating another success of the Cuban Science that Fidel began.”
In addition to Abdala, Soberana 02 and Soberana Plus, the Caribbean country is also developing two other vaccines: Soberana 01 of the IFV and Mambisa of the CIGB. All five vaccines are protein antigens that do not contain the live virus but contain part of the coronavirus. They work in a similar way and the difference between them is that each one has different formulations. The three Soberana vaccines and Abdala are injectable vaccines, administered by intramuscular route into the deltoid muscle, while Mambisa is a nasal spray vaccine. Soberana Plus is a booster shot for convalescents from COVID-19.
Last week, on June 14, Cuba also began the clinical trial of Soberana 02 and Soberana Plus in children between the ages of 3 and 18. Around 350 children and adolescents will participate in the trial.
In the face of 63 years of the US embargo against Cuba, the country’s achievements are extremely significant. The vaccine candidates are a proof of the power of Cuban biogenetic engineering and biotechnological advancements. Cuba began investing in biotechnology and medical science in the 1980s as part of Fidel Castro’s vision to make the nation self-sufficient in the face of the US blockade.
According to the recent data shared by the Cuban Public Health Minister, as of June 19, a total of 4,885,270 doses of Soberana 02 and Abdala have been administered. The data further elaborates that 2,244,336 Cubans have received at least one dose of one of the vaccine candidates, 1,765,242 people have received a second dose and 905,692 people have received a third dose.
Given the steep rise in COVID-19 infections in the country, Cuba needed to urgently roll out vaccines. After analyzing the phase-2 trial data of Abdala and Soberana 02 and establishing their safety for human use, the WHO allowed Cuba to begin the vaccine rollout in May.
As of June 20, the country has recorded 169,365 cases of COVID-19 and 1,170 deaths due to the disease. On June 20, 1,561 new cases and 11 deaths were reported. Presently, the country has 8,364 active cases, of which 8,203 patients are stable, while 161 patients are in critical and serious conditions.
Del mismo autor
- Progressive presidential ticket Petro-Márquez receives death threats in Colombia 06/04/2022
- Once again, right wing fails to impeach Peruvian President Pedro Castillo 30/03/2022
- Long before US extradition request, the streets of Honduras cried, ‘Get out JOH narco-dictator’ 17/02/2022
- Second Belmarsh Tribunal on US war crimes to be held in New York 15/02/2022
- Peruvian President swears in fourth ministerial cabinet in six months 09/02/2022
- Haiti’s National Transition Commission elects new interim president and prime minister 01/02/2022
- Colombia registers the 91st massacre of 2021 15/12/2021
- On Human Rights’ Day, UK court permits extradition of Julian Assange to US 10/12/2021
- State of siege in Guatemala is a threat to human rights 05/11/2021
- Global, moral, strategic leadership is urgent says PM of Barbados 05/10/2021
Pandemia
- Gabriela Ramírez Mendoza 07/02/2022
- Jyotsna Singh 06/02/2022
- Gabriela Ramírez Mendoza 06/02/2022
- Richa Chintan 10/01/2022
- Isaac Enríquez Pérez 03/01/2022
